Introduction Belantamab mafodotin (belamaf; GSK2857916), an antibody-drug conjugate targeting B-cell maturation antigen, has shown promising efficacy and a favorable tolerability profile in the treatment of multiple myeloma. Ocular adverse events (OAEs), manifesting as visual acuity changes, ocular symptoms, and corneal findings, are common with belamaf and the main reason for dose modifications (dose reduction, dose holds) or treatment discontinuation. Keratopathy and Visual Acuity (KVA) scale was used in most belamaf trials, including the pivotal DREAMM-2, to guide belamaf dose modifications. KVA grading is dependent on an ophthalmologist consultation prior to belamaf administration. Herein, we evaluate a novel approach to guide belamaf dosing in transplant ineligible (TI) patients (pts) with newly diagnosed multiple myeloma (NDMM), treated with an extended dosing schedule of belamaf in combination with lenalidomide and dexamethasone (Rd) in the phase 1/2 BelaRd study.
Methods BelaRd (NCT04808037) is an open-label, phase 1/2 study conducted in Greece, aiming to enroll 66 TI NDMM pts. Part 1 evaluated the safety/tolerability of three belamaf doses (2.5/1.9/1.4 mg/kg) plus Rd in 36 pts and established that the recommended phase 2 dose (RP2D) is 1.9 mg/kg, initially administered q8w and, depending on toxicity, adjusted to q12w. Part 2 investigates the safety/efficacy of RP2D in Groups A and B, comprised of 15 pts each, and evaluates two different sets of guidelines for the management of OAEs. In Group A, belamaf dosing is guided by the KVA scale; in Group B, dose modifications are determined by the pts' responses on ocular symptoms and their impact on activities of daily living (ADL), captured by the Vision Related Anamnestic (VRA) tool, and by the presence of ≥Grade (Gr) 3 KVA events (BCVA reduced/corneal findings). VRA is a novel, 9-item physician administered questionnaire designed to assess the frequency of ocular symptoms and their impact on vision-related activities. Ocular exams include Snellen best corrected visual acuity (BCVA) and slit lamp corneal evaluation, while ocular symptoms are classified by CTCAE v5.0. Herein, we present the OAEs and the clinical activity of the treatment combination for both Groups of Part 2 (cut-off date 05/06/2023).
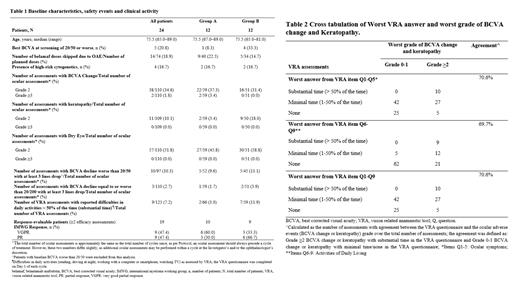
Results By the cut-off date, twenty-four pts [median age: 75.5 years; male: 16 (66.7%)] had received ≥ 1 belamaf dose in Part 2 and were still on treatment. At baseline, most pts had ocular comorbidities. The median belamaf administrations and number of cycles reached were 3.0/2.0 and 6.5/5.5, for Groups A and B, respectively. A meaningful BCVA decline (BCVA <20/50) with at least 3 lines drop in the better seeing eye, was noted in 5/52 (9.6%) and 5/45 (11.1%) assessments, while BCVA ≤20/200 with at least 3 lines drop was noted in 1/59 (1.7%) and 2/51 (3.9%), respectively. Overall, no ≥Gr 3 ocular symptoms were observed, while the most frequent ≥Gr 2 ocular symptom was dry eye (57/110, 51.8%); also, no ≥Gr3 keratopathies were recorded. Among 66/59 VRA assessments, ocular symptoms and ADL impairment, manifesting for >50% of the time (substantial time) in the last 24 hours prior to belamaf administration, were noted in 2 (3.0%)/9 (15.3%) and 2 (3.0%)/7 (11.9%), respectively. Importantly, numerically similar proportions of KVA events and skipped belamaf doses were noted in both Groups (Table 1). Furthermore, although the sample size of the present analysis does not allow conclusions for the VRA test characteristics, it is notable that in all assessments where eyesight difficulties were reported for substantial time, ≥Gr2 KVA events were also recorded, suggesting a potential association between VRA responses and KVA events, that warrants further investigation. Finally, in terms of clinical activity, for the 19 response-evaluable pts (≥2 efficacy assessments), at a median follow-up of 7.4 months the ORR was 90%/100% in Groups A and B (PR/VGPR: 30%/60%; 66.7%/33.3%) with no disease progression observed.
Conclusions In this preliminary descriptive analysis, the VRA tool was safe and effective in informing belamaf dose adjustments in the extended dosing schedule. Recruitment in Part 2 is ongoing and, as data accumulates, future analyses will provide further insight on the potential association of the VRA tool with KVA events, which may eliminate the need for an ophthalmic exam prior to belamaf dosing.
Disclosures
Terpos:BMS: Honoraria; ASTRA/Zeneca: Honoraria, Other: Travel Expenses; Amgen: Honoraria, Other: Travel Expenses, Research Funding; EUSA Pharma: Honoraria, Other: Travel expenses; Takeda: Honoraria, Other: Travel expenses, Research Funding; Sanofi: Honoraria, Other: Travel expenses, Research Funding; Pfizer: Honoraria; Menarini/Stemline: Honoraria; Janssen: Honoraria, Research Funding; GSK: Honoraria, Research Funding. Gavriatopoulou:Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; X4 Pharmaceuticals: Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/Genesis: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees. Migkou:Glaxo Smith Klein: Honoraria; Janssen-Cilag: Honoraria; Integris Pharma: Honoraria. Gkolfinopoulos:Heads: Current Employment. Kastritis:Janssen: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Sanofi: Honoraria; Pfizer: Honoraria, Research Funding. Dimopoulos:BeiGene Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal